Characterizing a novel target to combat obesity
Obesity has reached epidemic levels worldwide. It is a major cause of deadly illnesses, such as diabetes, cardiovascular disease, and some cancers, and has been linked to reduced quality of life and poor mental health outcomes. Efforts to combat this epidemic have so far failed to have an appreciable impact.
Seeking novel targets with the potential to move the needle on obesity-linked cardiometabolic disorders, a research team led by Marc Prentki and S. R. Murthy Madirajua at the Université de Montréal discovered glycerol-3-phosphate phosphatase (G3PP) in mammalian cells, an enzyme involved in the glycerolipid/fatty acid (GL/FA) cycle. This cycle is comprised of lipogenesis and lipolysis segments; it produces building blocks for complex lipids, such as triglycerides, promotes thermogenesis, and generates signaling molecules involved in many biological processes, including insulin secretion and action. Dysregulation of the GL/FA cycle is associated with metabolic and age-related diseases, including obesity and type 2 diabetes.
The MBF WormLab® software and imaging system was an integral part of this research. “Using Wormlab, a user-friendly and powerful software, we were able to track and analyze the locomotion behavior of a large pool of aging C. elegans nematodes. Wormlab enabled the quick and efficient analysis of recorded videos and the accurate quantification of multiple parameters including body bending, speed, worm length and others.” Elite Possik, PhD.
The authors explain that, prior to their discovery in 2016, mammals were thought to lack an enzyme capable of converting glycerol-3-phosphate (Gro3P) to glycerol. But according to the paper, G3PP can do just that, effectively bypassing lipolysis, which was previously thought to be the only source of glycerol in mammals. Their earlier research suggests a role for G3PP in “the control of glycolysis, gluconeogenesis, cellular redox, and energy production” in conditions of glucose excess.
 |
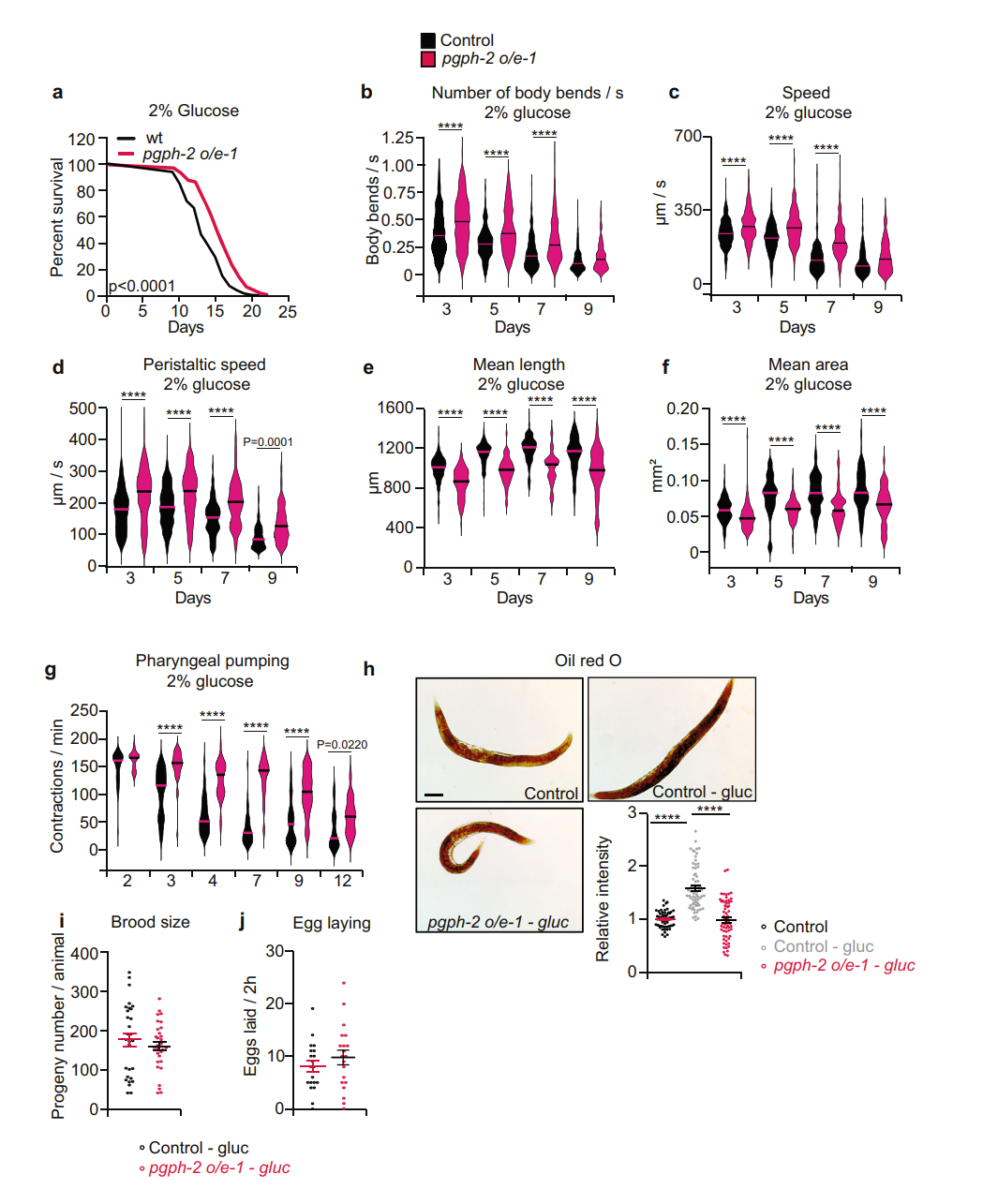 |
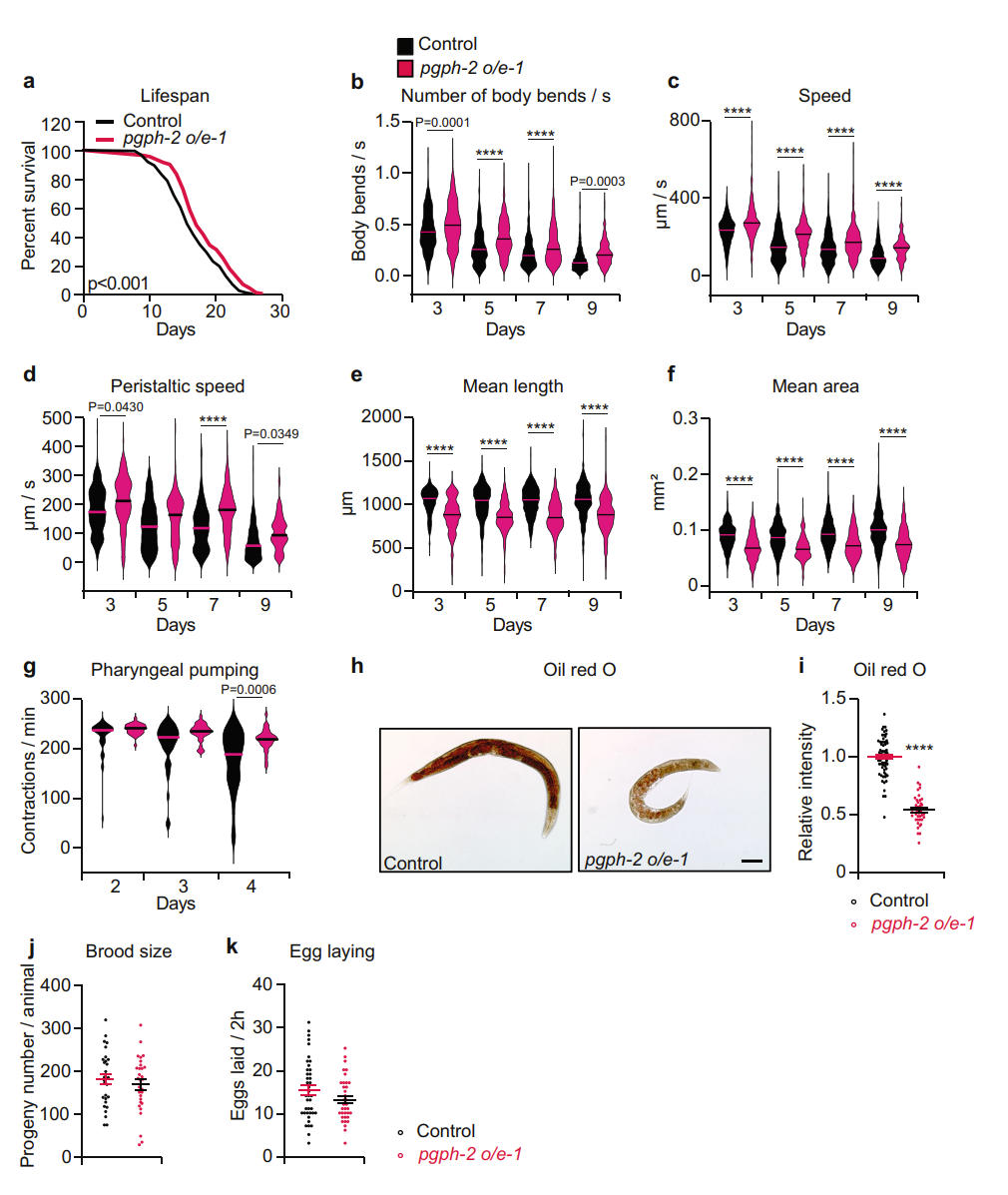
| Fig: 1 pgph-2 overexpression improves health span and decreases fat content. | Fig 2: pgph-2 overexpression increases lifespan under excess glucose and reduces glucose-induced fat deposition |
In this study, published in Nature Communications, the researchers used the model organism C. elegans to identify the biological functions of G3PP. First, they identified three homologs of mammalian G3PP in C. elegans based on protein sequence identity to the human enzyme; they’re named phosphoglycolate phosphatase homologs (PGPH), pgph-1, pgph-2, and pgph-3. Using triple pgph deletion mutants that lack all three homologs, the researchers established that the worm PGPH isoenzymes mimic G3PP in vivo under normal physiological conditions. They found no phenotypic defects in pharyngeal pumping, egg laying, brood size, or swimming behaviors, but did find that triple mutants had increased fat deposition in the context of normal levels of glycolytic metabolites such as, Krebs cycle intermediates, and DNA building and repair compounds. Using RNAi knockdown and expression analyses of genes known to drive lipogenesis in C. elegans, the team showed that the increased fat accumulation in triple mutants was due to an increase in the lipogenesis arm of the GL/FA cycle.
Response to hyperosmotic stress is of particular interest; glycerol is an important component of adaptation in many organisms. The researchers found that pgph genes were strongly induced in high-salt or high-glucose conditions. Deletion mutants were found to be significantly less able to tolerate these conditions compared to control worms. Further experiments demonstrated that both pgph gene expression and glycerol production increased when worms were exposed to 2% glucose, and loss of PGPH enzymes suppressed glucose-induced glycerol production. This supports the premise that PGPH enzyme activity “is required for resistance to hyperosmotic stress” from salt or excess glucose by increasing glycerol production.
Excess glucose has been shown to shorten the C. elegans lifespan. The research team extensively examined the roles and mechanisms of PGPH isoenzymes in susceptibility and resistance to glucotoxicity. The MBF Bioscience WormLab Imaging System was used to conduct lifespan and glucose toxicity assays. WormLab software enabled the scientists to film and analyze worms’ movement behaviors, which serve as an index of healthy aging.
Using pgph deletion mutants and RNAi knockdown, loss of PGPH activity shortened worm lifespan and healthspan in normal and high-glucose environments. The team then evaluated pgph-2 overexpression and found that it protected worms from glucose-induced toxicity, improved healthspan, decreased fat content, and extended median lifespan in glucose-rich conditions. Importantly, they showed that the decreased fat content was not due to decreased calorie intake and that the extended healthspan they observed was not at the expense of energy expended for reproduction. Further experiments with pgph-2 overexpressing worms revealed that, in the presence of glucose, worms maintained better locomotion behaviors with age. Specifically, they exhibited more body bends per second and higher locomotion and peristaltic speed. pgph-2 overexpressing animals’ fat content was lower than control worms in glucose-excess conditions and was comparable with that of control worms grown in normal growth medium; this correlated with decreased expression of lipogenesis genes in the pgph-2 overexpressors.
This study characterizes a key enzyme and a distinct biochemical pathway that mimics some of the beneficial effects of calorie restriction, without the drawbacks of fertility changes and the difficulty of maintaining reduced caloric intake. They conclude that G3PP activation has potential as a novel approach for prevention and treatment of diseases that occur from nutritional excess, such as obesity, diabetes, and cardiovascular disease.
Possik, E., Schmitt, C., Al-Mass, A. et al. Phosphoglycolate phosphatase homologs act as glycerol-3-phosphate phosphatase to control stress and healthspan in C. elegans. Nat Commun 13, 177 (2022). https://doi.org/10.1038/s41467-021-27803-6
Learn more about the WormLab Imaging System, a complete, scalable solution for automated imaging and quantitative analysis of behavior in C. elegans and other nematodes.
Stand-alone WormLab software is also available for imaging, tracking, and behavior analysis in C. elegans and other worms. Learn more about WormLab software here.


