
Neurolucida Helps Florida Researchers Reconstruct a Region of the Rat Brain
Scientists at the Max Planck Florida Institute are studying the functional responses of neurons in the rat vibrissal cortex. Using a “pipeline” method, developed to use data obtained from animals to recreate parts of the brain “in silico” (1), they have constructed a 3D model of a vibrissal cortical column. The scientists used Neurolucida® to trace neurons so they could be classified according to dendritic morphology and cell body location.
In their paper (2) “Cell Type-Specific Three-Dimensional Structure of Thalamocortical Circuits in a Column of Rat Vibrissal Cortex,” the scientists classified nine cell types in the barrel cortex, a region of the vibrissal area of the rodent somatosensory cortex. They used these cell-types and parameters such as 3D cell location and quantity, spine and bouton densities, and definitions of pre and post-synaptic partners, to assemble an anatomically realistic network that included synapses at points where boutons and spines overlapped. Though incomplete, this preliminary model shows that thalamocortical connectivity is correlated with cell type and 3D soma location, and that these cell types have some correlations with spiking patterns.
The rat’s sensory process relies on a sophisticated network of neuronal communication. Primary sensory neurons “pick up” a signal from a whisker-deflection and synapse on secondary sensory neurons in the brainstem, which in turn relay the signal to tertiary sensory neurons in the VPM nucleus of the thalamus. The target of the information from the thalamus is the main sensory cortex. The information from one whisker is thought to end up in an anatomically recognizable region of the vibrissal area of the rodent somatosensory cortex, called a “barrel column.”
The authors reconstructed thalamic axons (n=12) and cortical neurons (n = 95), by using Neurolucida to trace cells that were labeled in vivo. Cortical and thalamic neurons were filled with biocytin in anesthetized rats and reconstructed from tens of 50 or 100 microns thick vibratome sections per neuron in order to visualize the soma and dendrites of the cortical neurons and the axons of the thalamic neurons. Average inter-bouton distance on the afferent thalamic axons (horizontally projecting axons were picked for analysis) was obtained from high-resolution image stacks. Distribution of spines varied within a given neuron, but was assumed to be a constant and uniform distribution for each cell type.
The cortical neurons were then classified into nine types based on morphology using a clustering algorithm. The first “run” used 90 different morphological features to objectively classify cells, eventually slimming to 21, including cell-body location and different aspects of dendrite morphology. The nine cell types and their location within the cortex-column are beautifully illustrated in figure 2 of the paper (2).
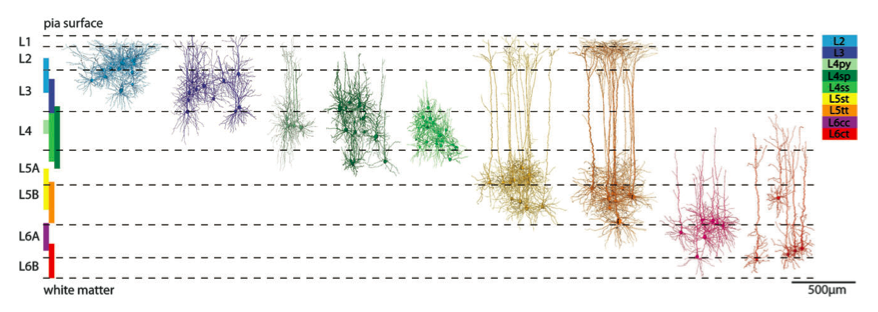
To reconstruct a cortical barrel column, the researchers first reconstructed the pial surface and barrel volume (550 X 550 X 2000 μm3) that provided a standardized reference frame to allow an average network to be generated from anatomical data from different animals. They used 3D confocal microscopy and automatic particle detection software to quantify and analyze neuron cell bodies in slices stained with NeuN. Based on this in-vivo data, the study showed around 15,000 excitatory somata within the cylindrical shaped model column, in silico.
Each neuron in the barrel was assigned one of the nine cell types; the assigned reconstruction had to be of the same cell type called for by the soma location. To be eligible for insertion, the authors decided that a reconstruction could not be located more than 50 microns away from the computed cell body. Reconstructions were rotated around the z-axis until their positioning resembled their original orientations in the barrels they were taken from. For instance, reconstructions of cells on the periphery of the barrel, with dendrites polarized towards the center, were rotated in the standardized barrel to make sure their dendrites pointed inwards.
The model of the circuit diagram sheds light on its function. The researchers obtained estimates for the numbers and 3D distributions of somas, dendrites, and synapses. They found that somas and dendrites of cell types intermingled within and across cytoarchitectonic layers, and that the cell type-based boundaries compliment but differ from the neuron density-based cytoarchitectonic layer boundaries. They also determined that structural overlap shows the amount and cellular location of synapses is cell type and location specific, and the number of potential synapses per neuron decreases for all cell types as the distance of the cell from the center of the barrel increases.
Some of the morphologically reconstructed barrel cortex neurons were recorded to determine electrophysiological parameters in the anesthetized rat (3), as were the spiking rates of cell type specific, spontaneous, and whisker-evoked neurons. The researchers found some correlations between cell type specific physiology and anatomical parameters. For instance, they saw a high correlation between spontaneous spiking frequencies and total dendritic length. The study also showed a correlation between whisker-evoked spiking and number of putative synapses for some cell types.
We are pleased that Neurolucida is playing a role in the pipeline to model brain circuits in silico. As more is added to the model, for instance inhibitory neurons, it will become a more powerful tool for understanding the cellular and subcellular mechanisms underlying function. The pipeline (1) also allows for physiology to be added into the mix; passive membrane parameters and active conductances can be given to the neurons. This will allow activation patterns, also based on in-vivo experiments, to be used to stimulate this recreated circuit, in silico, to help study the underlying mechanisms of its function.
- Lang, S., V.J.Dercksen, B. Sakmann, and M. Oberlaender, 2011, Simulation of signal flow in 3D reconstructions of an anatomically realistic neural network in rat vibrissal cortex. Neural Networks, doi:10.1016/j.neunet.2011.06.013
- Oberlaender M., C.P.J. de Kock , R.M. Bruno, A. Ramirez, H.S. Meyer, B.J. Dercksen, M. Helmstaedter, and B. Sakman, 2011, Cell Type-Specific Three-Dimensional Structure of Thalamocortical Circuits in a Column of Rat Vibrissal Cortex. Cerebral Cortex, doi: 10.1093/cercor/bhr317’
- De Kock, C.P.J., R.M. Bruno, H. Spors, and B. Sakman, 2007, Layer- and cell-type-specific suprathreshold stimulus representation in rat primary somatosensory cortex. J Physiol. 581.1, pp 139-154.
Oberlaender, M.1,2, de Kock, C.P.2, Bruno, R.M., Ramirez, A., Meyer, H.S., Dercksen, V.J., Helmstaedter, M., and Sakmann, B. Cell Type-Specific Three-Dimensional Structure of Thalamocortical Circuits in a Column of Rat Vibrissal Cortex. Cereb Cortex. Epub 2011 Nov 16.



