
How Transplanted Stem Cells Behave in Injured Spinal Cord Tissue
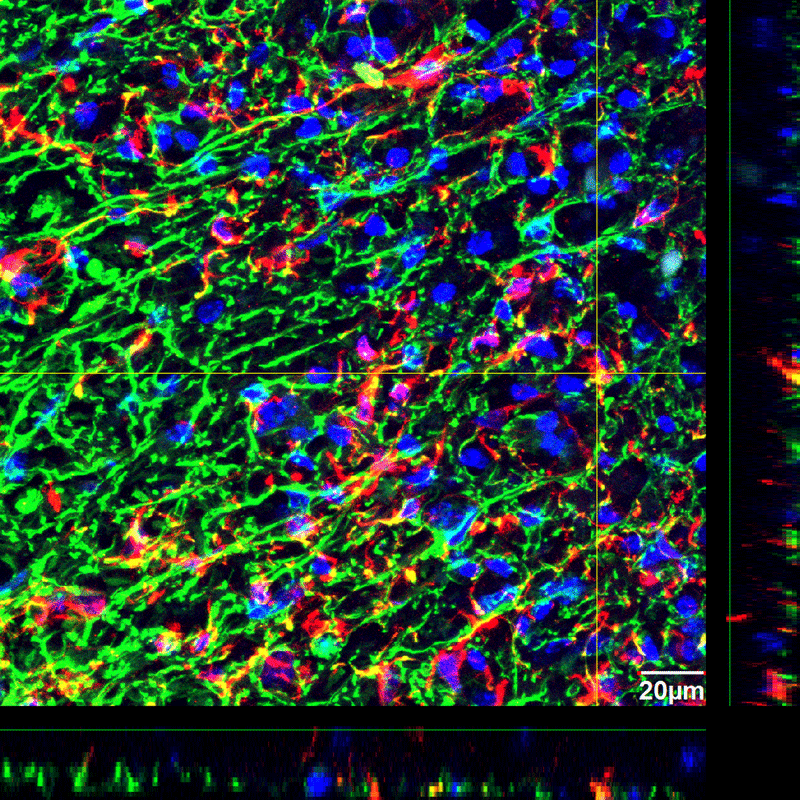
A representative confocal image of spinal cord tissue fluorescently immunolabeled for SC121 (red) in conjunction with GFAP (green) – markers that allowed researchers to quantify stem cell differentiation and migration. (Image provided by study author Dr. Aileen J. Anderson)
Research has shown that transplanting human neural stem cells into damaged spinal cords restores locomotor function in a mouse model of spinal cord injury1. Researchers who worked on that study have published another paper examining how these neural stem cells behave in injured tissue as they aid in healing. Learning how stem cells behave in injured tissue will hopefully help researchers develop better treatments for spinal cord injuries.
In the study, researchers used Stereo Investigator to stereologically quantify the survival, migration, proliferation, and differentiation of human neural stem cells transplanted into injured and uninjured mice. Stem cells were analyzed in mouse brain tissue specimens 1, 7, 14, 28, and 98 days after transplantation. The research found that there were fewer stem cells in the injured animals compared to the uninjured animals at all time points, stem cells in injured mice localized near the center of the injury, a delay of stem cell proliferation in injured tissue led to an overall deficit of actively dividing cells, proliferation in injured mice occurred closer to the injection sites (the locations where the stem cells were injected into the mice), and the injured microenvironment increased differentiation to more mature oligodendrocytes.
SC121, a marker that binds to human cytoplasm in cells, was used to identify human neural stem cells in the tissue sections of mouse spinal cord. The researchers stereologically quantified this marker to find the number of human neural stem cells in the injured and uninjured tissue at each time point. Analysis at one day post-transplantation showed that only 11% of the initial transplant dose survived in injured animals while 25% survived in uninjured animals. The number of stem cells in each group increased over time, however, there were fewer stem cells in the injured animals compared to the uninjured animals at all time points.
To analyze the migration of stem cells, researchers used Stereo Investigator to quantify the number of stem cells at varying distances from the injection sites (the locations where the stem cells were injected into the mice), and expressed these localized counts as percentages of the total number of cells counted. At 98 days post transplantation there was a significant difference in the distribution of stem cells between injured and uninjured spinal cords; stem cells in injured mice localized near the center of the injury.
Proliferation was quantified by using Stereo Investigator to count the number of cells that express SC121 and BrdU or KI67 – an indication that cells are in the process of dividing. Very few cells were actively dividing 1 day post transplantation and there was no significant increase in the number of actively dividing cells in injured tissue specimens, except when comparing 28 days post transplantation data to 1 day post transplantation. This delay of stem cell proliferation in the first 28 days in injured tissue led to an overall deficit of actively dividing cells throughout the entire period. Also interesting is that with all of the cell division activity, there were no tumors detected.
Then, the researchers wanted to see where proliferation was occurring and where the stem cells migrated to. They quantified this by taking the numbers of cells expressing SC121 and BrdU at given distances from the site of injection and expressing them as percentages of the total number of cells counted. Proliferation in injured mice occurred closer to injection site and prior to migration toward the injury epicenter, while stem cells in the uninjured spinal cord proliferated in multiple waves alternating with migration along the length of the spinal cord.
To find out what type of cells the adult stem cells differentiated into, the researchers stereologically quantified stem cells that expressed markers for oligodendrocytic (OLIG2), astrocytic (SC123) and neuronal (DCX) lineages at 98 days post transplantation. They found that the injured tissue had more oligodendritic cells than the uninjured tissue and overall the majority of stem cells differentiated along oligodendrocytic lineage.
Migration and distribution of the differentiated cells were examined at 98 days post transplantation. The researchers found that stem cells along the oligodendrocytic lineage were in localized peaks around the injury epicenter, as opposed to an even distribution along the length of the spinal cord as found in the uninjured tissue. There also was a significant increase in the percentage of SC123 stem cells in injured animals at the center of the injury that was directly proportional to the decrease of OLIG2 cells. This suggests that astrocytes were recruited to the injury site at the expense of oligodendrocytes.
The researchers noticed that many of the stem cells did not have a marker to indicate what type of differentiated cell it is. Since OLIG2 is a marker for precursor/progenitor oligodendrocytes, researchers postulated that these unmarked cells could be older oligodendrocytes. So they used APC/CC1 – a marker for more mature oligodendrocytes. When they did this, the percentage of cells accounted for increased from 71.9% to 97.4%. This suggests that, on the whole, the injured microenvironment increased differentiation to more mature oligodendrocytes.
Stem cell treatments have emerged as an option to help repair nerve damage suffered from spinal cord injuries, however there is still much to be learned about stem cells and how to effectively use them to treat spinal cord injuries. The stereological data and analyses reported in this research study uncover interesting information about how human neural stem cells behave in a mouse model of spinal cord injury: fewer stem cells survived in injured tissue and proliferation was delayed – causing a deficit in the number of stem cells through the length of study. Stem cells proliferated near the injection site and then migrated toward the injury, and they differentiated into more mature oligodendrocytes. Hopefully, the information presented in this study can be used to help find more effective treatments for spinal cord injuries.
1Cummings, B.J., Uchida, N., Tamaki, S.J., Salazar, D.L., Hooshmand,M., Summers, R., Gage, F.H., and Anderson, A.J. (2005). Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl. Acad. Sci. USA 102,14069–14074.
Sontag, Christopher J., Uchida, N., Cummings, Brian J., & Anderson, Aileen J. (2014). Injury to the Spinal Cord Niche Alters the Engraftment Dynamics of Human Neural Stem Cells. Stem Cell Reports(0).


