Researchers Observe Altered Neuropathology in CTE Brains
Researchers at Colorado College may have identified a new neuropathological hallmark of Chronic Traumatic Encephalopathy (CTE). Their findings, published in the Journal of Comparative Neurology, describe overall dendritic atrophy across cortical neurons and greater morphological variability in CTE brains compared to controls.
A neurodegenerative disorder characterized by late-onset symptoms like depression, confusion, and memory loss, CTE is caused by repeated impacts to the brain. The disease is most often seen in athletes who play high impact sports and military veterans.
While CTE is known to be associated with abnormal aggregations of the tau protein, which is also observed in diseases like Alzheimer’s disease and traumatic brain injury, no research to date had explored how CTE affected neuron morphology.
In an attempt to get a glimpse into the neurodegenerative mechanisms at work in the CTE brain, researchers at Colorado College quantified the dendritic arborizations of supragranular pyramidal neurons in two regions of the human brain — the frontal and occipital lobes.
Using Neurolucida to reconstruct Golgi-stained neurons from these two regions in 16 human brains (11 cases with CTE and 5 control cases without the disease), a team of researchers led by Dr. Bob Jacobs, observed a general degeneration and greater variability between dendritic systems in CTE brains compared to controls.
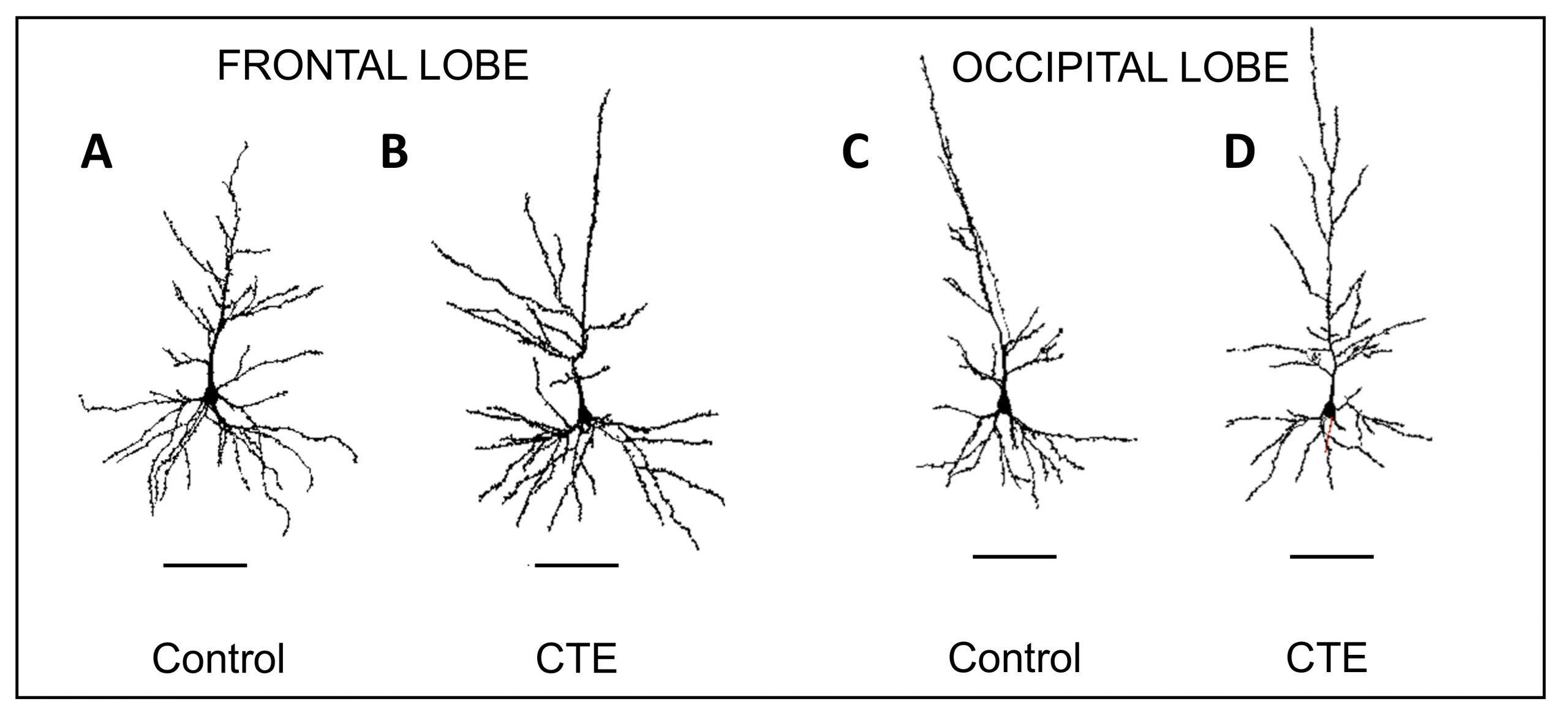
Representative Neurolucida tracings of pyramidal neurons in the frontal and occipital lobes from control and chronic traumatic encephalopathy (CTE) groups. CTE tracings show greater variability in dendritic extent compared to control tracings.
According to the study, the dendritic systems of CTE brains showed reductions in seven different measures — dendritic volume, total dendritic length, mean segment length, dendritic segment count, dendritic spine number, and dendritic spine density, as well as dendritic diameter. In contrast to the occipital lobe, dendritic apathy and loss was more severe in the frontal lobe, where p-tau is known to aggregate, possibly acting as a mechanism associated with this pathology, according to the paper.
The researchers also observed a greater variability of morphological differences between dendritic systems in the CTE brains as compared to controls, which were more uniform in size and branching patterns. As outlined in the paper, this variability among neurons in CTE brains might be a result of a combination of the disease’s degenerative effects as well as reorganizational efforts to compensate for the damage.
“This study is the first to document changes in cortical dendritic systems in CTE subjects, which sheds light on an entirely different realm of CTE related neural alterations,” said Dr. Jacobs. “These dendritic changes are probably associated with at least some of the cognitive changes one sees in CTE as well.”
While the authors point out that dendritic degeneration is a normal process in the aging brain, they also explain that their observations of CTE brains in comparison to similarly aged non-CTE brains reveal a possible acceleration in dendritic loss when CTE is present.
Over the course of their study, the scientists traced 640 Golgi-stained neurons with Neurolucida.
“Neurolucida was very effective for this study. The software has come a long way in the last 30 years and has become much easier to use,” said Dr. Jacobs, who, over the course of his career, has traced over 5,000 neurons across 26 different species with the help of Neurolucida.
“I would not have had a research career without the Neurolucida system and the wonderful support of MBF over the years,” he said.


