Neurolucida Helps Look at Whether Dendrites Can Tell Inputs Apart
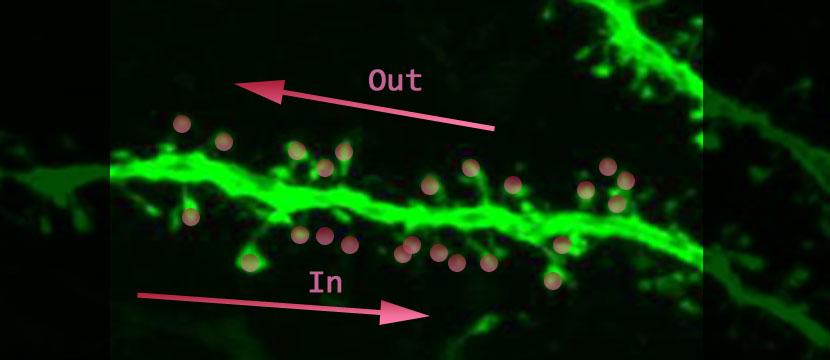
What would you do with a neuron if you could activate its synapses in any combination you wanted? Tiago Branco, Beverley A. Clark and Michael Hauser created a chance to do just that (Branco, 2010). The authors, using in-vitro brain slices containing layer II/III pyramidal cells in visual or somatosensory cortex of rats, were able to excite identified spines in any order and with whatever timing. They activated the synapses in one direction and then in the other direction (above is a dendrite not from this study; “In” is the direction towards the cell body, and “Out” is the direction away from the cell body) to see if the output-signal of the cell would be different. Along the way they collected more evidence that signal integration happens at the level of the dendrite. The most exciting result is that the output-signals generated in the soma are dependent on the order that the spines were activated.
To study the oblique radial dendrite of the cortical pyramidal cell, one of the smallest dendrites in the brain, multi-site two-photon glutamate uncaging (Judkewitz, 2006; Losonczy, 2006) was used, achieving exquisite control of which spines will be activated when. The idea is to keep the excitatory neurotransmitter, glutamate, hanging around in an inactive form (represented as pink circles above). Photons are used to both convert the glutamate to its active form and to observe the fluorescently-labeled tissue. The amount of glutamate released is believed to only affect one spine, and the time course is such that it can be used to approach physiological conditions. The spines on the dendritic branch can be activated with a spatial and temporal pattern of the authors’ choosing; and the resulting voltage change that can be thought of as the output-signal of the cell, is recorded with an intracellular electrode at the cell body (see the figure below graciously provided by the authors).
 Why is the order and timing of synapse activation worth looking at? A neuron functions to collect from the axons of other neurons signals that cause voltage changes in its dendrites; and to pass these signals along to its cell body and axon where the voltage-threshold for an axonal action potential (AP) might or might not be reached. Features of stimuli in sensory pathways can be coded for by the timing of dendritic excitation. One example is in the retina, where individual dendritic branches of retinal starburst amacrine cells show directionally selective signals (Häusser, 2003; Euler, 2002). The authors (Branco, 2010) also point out that temporal and spatial variability in dendritic excitation patterns is especially relevant for circuits with layered input, like the hippocampus, where it could be used by dentate gyrus granule cells to directly detect the sequence of entorhinal cortex activation. Integrative properties of the dendrites appear to be at least one mechanism that can differentially encode spatial and temporal synchrony.
Why is the order and timing of synapse activation worth looking at? A neuron functions to collect from the axons of other neurons signals that cause voltage changes in its dendrites; and to pass these signals along to its cell body and axon where the voltage-threshold for an axonal action potential (AP) might or might not be reached. Features of stimuli in sensory pathways can be coded for by the timing of dendritic excitation. One example is in the retina, where individual dendritic branches of retinal starburst amacrine cells show directionally selective signals (Häusser, 2003; Euler, 2002). The authors (Branco, 2010) also point out that temporal and spatial variability in dendritic excitation patterns is especially relevant for circuits with layered input, like the hippocampus, where it could be used by dentate gyrus granule cells to directly detect the sequence of entorhinal cortex activation. Integrative properties of the dendrites appear to be at least one mechanism that can differentially encode spatial and temporal synchrony.
The sensitivity of single dendrites to the order of activation of a defined set of synapses was tested. When activated in isolation, the glutamate excitatory post-synaptic potentials, measured with an intracellular electrode at the cell body, were within physiological range. When the same spines along the dendrite were activated sequentially instead of in isolation, the IN direction always produced a larger somatic voltage response than the OUT direction, and this went along with a bigger chance for an axonal AP. Calcium signals were also larger in the IN than in the OUT direction. The most effective speed to show direction sensitivity was 2.6 microns per second. The dendrite itself can signal the difference between inputs that travel along it in one direction or the other!
What is going on in the dendrites that would cause activation of spines in one direction to give a different output-signal than activation of spines in the other direction? One idea is that dendrites of a neuron see all synapses as equal, and the voltage changes of the membrane caused by the synapses are summed linearly at the axon, possibly resulting in an axonal AP if the threshold is reached. But if they are all equal, and simply summed, the order of activation shouldn’t matter. Another idea is that the dendrites have active conductances, which would result in non-linearities (Häusser, 2003; Losonczy, 2006; Larkum, 2007). Non-linearity means that the whole is different than the sum of its parts; a supralinearity is the situation where the response when the identified synapses are activated sequentially is greater than the sum of the voltage responses from the same synapses activated in isolation. Regenerative events in dendrites are responsible for non-linearities in pyramidal neurons (Schaeffer, 2003); the axonal AP is back-propagating into the dendrites and long-lasting, mainly Ca2+ mediated depolarizations are initiated in the distal regions of apical dendrites. The distal depolarizations are an example of forward propagation (Vetter, 2001). The ability of thin dendritic branches of pyramidal neurons to support forward propagation called a ‘dendritic spike’ has been known for some time. These dendritic spikes are carried by Na+, Ca2+ and predominantly by special glutamate conductances mediated by NMDA receptors (Judkewitz, 2006). In this study, the voltage responses at the cell body were supralinear, meaning if you add together the individual synaptic responses from spines that are activated in isolation, the amplitude is smaller than if the same spines are activated sequentially. Something is boosting the signal to cause the supralinearity. This effect develops gradually with increasing numbers of recruited synapses. When the NMDA Glutamate receptor was blocked, the supralinearity disappeared, and furthermore, direction sensitivity, velocity sensitivity, and detectable dendritic calcium signals were abolished. This evidence points to amplification via NMDA-dependent regenenerative signal boosting and NMDA dendritic spikes.
Where does our program, Neurolucida, come in? The best research uses multiple techniques; and the authors decided to use Neuron, an electrophysiological modeling program, to study the conductances that have been implicated in creating the voltage non-linearities. The neurons were traced using Neurolucida. Neuron has a feature to import Neurolucida tracings. This way the anatomical arrangement of the dendrites is used in the electrophysiological modeling program; the authors could pick one dendritic branch, virtually activate its synapses either in isolation or in sequence, and look at the response at the cell body. Direction sensitivity could be reproduced with a simple model using dendrites with passive electrical properties and synapses containing AMPA and NMDA conductances. The NMDA conductance starts out small and gets bigger over time for the OUT direction and starts out large and gets smaller over time for the IN direction. Direction and velocity sensitivity are abolished by leaving only AMPA receptors and removing the NMDA receptors. There is asymmetric recruitment of NMDA receptors when activating synapses in the different directions. This is due to the smaller input resistance at the tip of the dendrite combined with the highly nonlinear voltage dependence of the NMDA receptor conductance.
So picture it this way. A pyramidal neuron in the sensory cortex is firing axonal APs in response to some sensory stimulus. These APs back propagate into the dendrites. Along with the back propagation the dendrite also experiences forward propagation as a result of active conductances that create a dendritic spike. The back propagation will be maintained or attenuated by the nature of the geometry of the dendritic tree (Schaefer, 2003; Vetter, 2001). Now what if one sensory stimulus sequentially activates the spines along a dendritic branch in the IN direction and another activates it in the OUT direction. For the IN direction, the first synapse activated is at the tip of the dendrite. How is this different than when the first synapse is at the base of the dendrite? First of all, due to differences in location along the geometry of the dendritic tree, the back-propagation voltage signal will be different. Also the dendrites taper, so the tip will have less radius and a greater input resistance than the base. Therefore, the history of what happened to each synapse is different depending on the IN or OUT direction. The NMDA receptors on the dendrites have a non-linear voltage dependence, so the different history or the differences in what just happened to the neighboring synapse, causes a larger signal for the IN than for the OUT direction. The dendrite itself can detect the difference between the two sensory stimuli. The evidence gathered from this work supports the exciting and important conclusion that these cortical neurons use their dendrites to not just pass the signal on, but to change the signal; and furthermore to change the signal based on the time and space pattern of the input to its synapses.
Branco T., Clark B. A., & Häusser M., 2010, Dendritic discrimination of temporal input sequences in cortical neurons. Science, 329, pp. 1671 – 1675.
Euler T, Detwiler, P.B., & Denk W., 2002, Directionally selective calcium signals in dendrites of Starburst Amacrine Cells. Nature, 418, pp. 845 – 852.
Häusser M. & Mel B., 2003, Dendrites: bug or feature? Current Opinion in Neurobiology, 13, pp. 372 – 383.
Judkewitz B., Roth A., & Häusser M., 2006, Dendritic enlightenment: using patterned two-photon uncaging to reveal the secrets of the brain’s smallest dendrites. Neuron, 50, pp. 180 – 183.
Larkum M.E., Waters J., Sakmann B., & Helmchen, F., 2007, Dendritic spikes in apical dendrites of neocortical layer 2/3 pyramidal neurons. Journal of Neuroscience, 27, pp. 8999 – 9008.
Losonczy A. & Magee J.C., 2006, Integrative properties of radial oblique dendrites in Hippocampal CA1 Pyramidal Neurons. Neuron, 50, pp. 291 – 307.
Schaefer A.T., Larkum M.E., Sakmann B., & Roth A., 2003, Coincidence detection in pyramidal neurons is tuned by their dendritic branching pattern. Journal of Neurophysiology, 89, pp. 3143 – 3154.
Vetter P., Roth A., & Häusser M. 2001, Propagation of action potentials in dendrites depends on dendritic morphology. Journal of Neurophysiology, 85, pp. 926 – 937.